
I. Introduction: The Allure of the Crunch
There's an undeniable, almost primal satisfaction that comes with biting into a perfectly crispy piece of fried food. That audible crunch, the delicate shattering of the exterior, and the tender, flavorful interior create a sensory experience that transcends mere sustenance. From golden-brown french fries and succulent fried chicken to delicate tempura and crispy spring rolls, fried foods hold a special place in global cuisine and in the hearts of countless eaters. But what exactly is it about that crispy exterior that makes it so irresistible? Is it simply a matter of technique, or is there a deeper scientific explanation at play?
While the art of frying has been perfected over centuries through trial and error in kitchens worldwide, the true magic behind achieving that coveted crispiness lies in a fascinating interplay of physics and chemistry. It's a delicate dance between hot oil, moisture, starches, and proteins, orchestrated by precise temperatures and timing. Understanding these underlying scientific principles can transform a hit-or-miss frying experience into a consistently perfect crunch, elevating your culinary skills from intuition to informed mastery.
This article will delve into the intricate science behind the irresistible crispiness of fried foods. We will explore the key reactions and techniques that contribute to this beloved texture, from the dramatic escape of moisture and the complex flavor development of the Maillard reaction to the structural changes in starches and the crucial role of the frying medium. By demystifying the science, we aim to empower you to achieve perfectly crispy results every time, turning your kitchen into a laboratory of delicious discovery.
II. The Role of Moisture: The Great Escape
At the heart of every crispy fried food lies a dramatic transformation involving moisture. When food is submerged into hot oil, typically between 325°F and 375°F (160°C and 190°C), a rapid and violent process of water displacement begins. The intense heat of the oil instantly causes the surface moisture within the food to boil and vaporize. This phenomenon is often visible as vigorous bubbling around the food, which is essentially water turning into steam and escaping into the oil.
This rapid evaporation of surface moisture is crucial for crispiness. As water leaves the food, it creates a dry, porous outer layer. This dry layer is what will eventually become the crispy crust. The steam generated also plays a vital role; it creates an outward pressure that pushes against the incoming oil, effectively preventing the food from becoming greasy and soggy. This protective barrier of steam ensures that the oil primarily cooks the food from the outside, rather than saturating it.
The golden rule for maximum crispiness is to minimize initial moisture. Excess surface water on food acts as a barrier to direct heat transfer and can significantly lower the oil's temperature, leading to a longer cooking time and a less crispy, more oily result. This is why patting food dry with paper towels before frying is a universally recommended step. For foods with high internal moisture, like potatoes, blanching them first can help reduce water content and promote a crispier outcome. The more efficiently moisture can escape and be replaced by a dry, rigid structure, the crispier the final product will be.
III. The Maillard Reaction: Flavor and Color Development
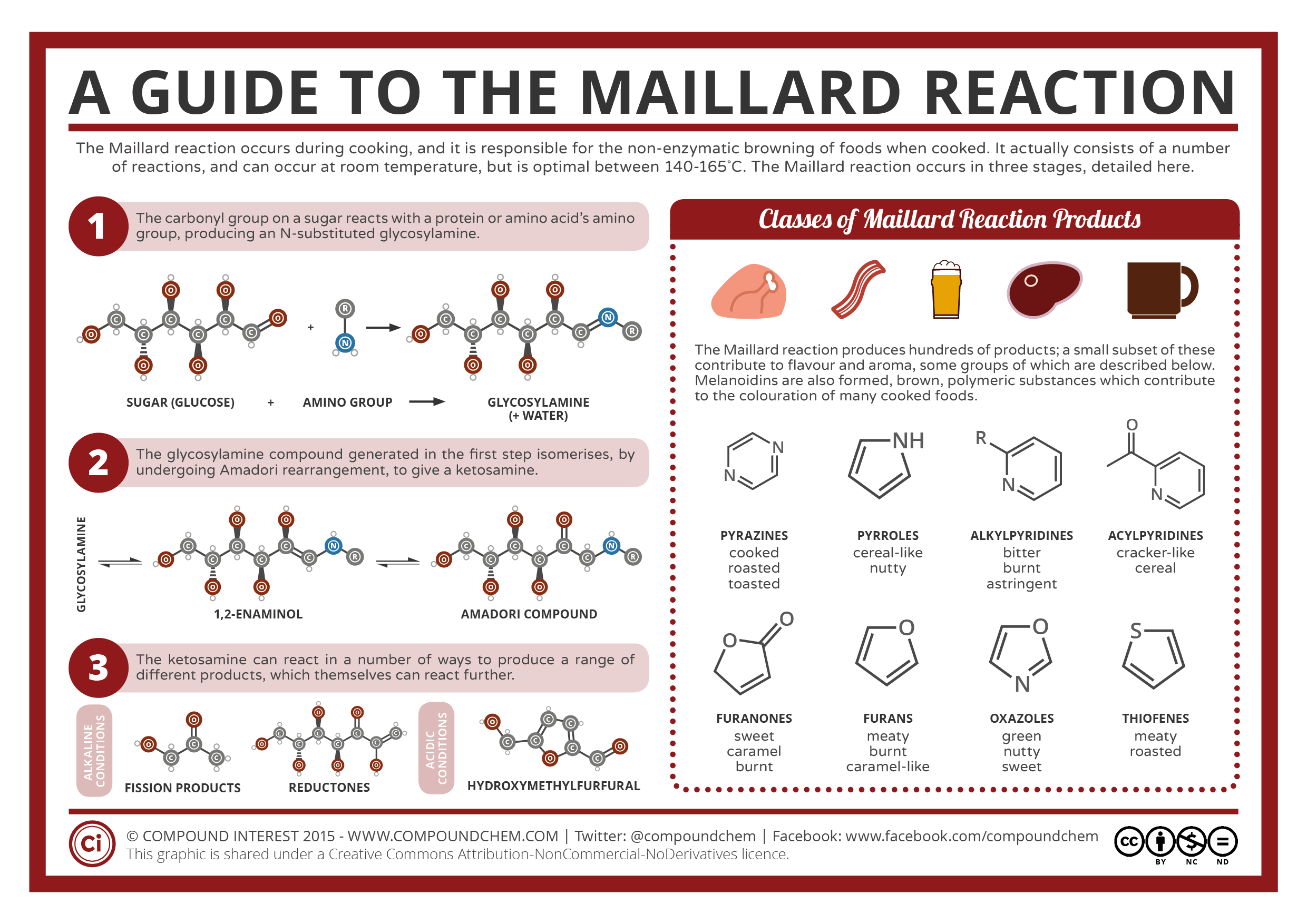
Beyond the satisfying crunch, perfectly fried foods are also characterized by their rich, complex flavors and appealing golden-brown color. Much of this sensory delight is attributed to a fascinating chemical process known as the Maillard reaction. This non-enzymatic browning reaction occurs when amino acids (the building blocks of proteins) and reducing sugars (simple sugars like glucose and fructose) react together under heat.
Unlike caramelization, which involves only sugars, the Maillard reaction is responsible for the vast array of flavors and aromas developed in many cooked foods, including seared steaks, roasted coffee beans, baked bread crusts, and, of course, fried foods. As the surface of the food heats up during frying, the Maillard reaction kicks into high gear, typically occurring rapidly at temperatures between 280°F and 330°F (140°C and 165°C). This complex cascade of chemical changes produces hundreds of new flavor compounds, contributing to the savory, nutty, roasted, and even slightly sweet notes that make fried foods so appealing. These compounds also contribute to the desirable golden-brown crust, which is a visual indicator of deliciousness.
Achieving the perfect balance of flavor and color from the Maillard reaction requires careful control of temperature and time. If the oil is too hot, the exterior of the food will brown too quickly, potentially burning before the interior is cooked through, and leading to a bitter, acrid taste. If the oil is too cool, the Maillard reaction will proceed too slowly, resulting in a pale, less flavorful crust and potentially greasy food as it absorbs more oil. Maintaining the optimal oil temperature ensures that the Maillard reaction occurs at the right pace, creating a rich, flavorful crust without compromising the texture or internal doneness of the food. This delicate balance is key to unlocking the full sensory potential of fried dishes.
IV. Starch Gelatinization and Dextrinization: The Crispy Skeleton
Beyond the dramatic expulsion of moisture and the flavorful browning of the Maillard reaction, the structural integrity of a crispy fried food often relies heavily on the behavior of starches. Most fried foods, whether naturally starchy like potatoes or coated with batters and breadings, utilize the unique properties of starch to form their characteristic crispy skeleton.
A. Starch in Coatings
Flour, cornstarch, rice flour, and other starchy components are fundamental ingredients in batters and breadings designed for frying. These starches, when exposed to heat and moisture, undergo a process called gelatinization. As the food fries, the water within the food and the batter/breading heats up. At a certain temperature, the starch granules absorb this hot water, swell, and eventually burst, releasing amylose and amylopectin molecules. These molecules then form a viscous, gel-like network that traps moisture and provides structural support. This gelatinized starch network is the initial foundation of the crispy crust.
B. Gelatinization
As frying continues and more moisture evaporates, the gelatinized starch network begins to dry out and rigidify. Simultaneously, another process called dextrinization occurs. Dextrinization is the breakdown of starch molecules into smaller carbohydrate chains called dextrins, which are simpler sugars. This process contributes to the golden-brown color of the crust and also adds a subtle sweetness and nutty flavor. The formation of these dextrins, coupled with the continued dehydration, creates a brittle, glassy structure that is the essence of crispiness.
C. Gluten Development (or lack thereof)
For many crispy fried applications, particularly those involving batters and breadings, minimal gluten development is desired. Gluten, a protein found in wheat flour, forms an elastic network when hydrated and kneaded. While essential for the chewy texture of bread, excessive gluten development in a fried coating can lead to a tough, rubbery, or bready texture rather than a light, crisp one. This is why many recipes for crispy fried foods call for low-protein flours, or incorporate starches like cornstarch or rice flour, which are naturally gluten-free and contribute to a more delicate, shattering crispness. The goal is to create a rigid, porous structure that shatters easily, rather than a chewy one that resists breakage.
V. The Fat Factor: Choosing the Right Medium
The oil used for frying is far more than just a heat transfer medium; it is a critical component that significantly influences the crispiness, flavor, and overall quality of fried foods. Selecting the right oil and maintaining its optimal condition are paramount to achieving perfect results.
A. Oil Selection
When choosing a frying oil, several factors come into play: smoke point, flavor neutrality, and stability. The smoke point is the temperature at which an oil begins to break down and produce smoke. Frying oils should have a high smoke point (above 375°F or 190°C) to withstand the high temperatures required for frying without burning or imparting off-flavors. Oils like peanut oil, canola oil, sunflower oil, and vegetable oil are popular choices due to their high smoke points and relatively neutral flavors, which allow the natural taste of the food to shine through. Oils with lower smoke points, such as extra virgin olive oil or butter, are unsuitable for deep frying as they will burn quickly and impart an unpleasant taste.
Flavor neutrality is also important. While some oils, like sesame oil, have distinct flavors that can be desirable in certain dishes, for general frying, a neutral oil ensures that the flavor of the food itself is the star. Stability refers to an oil's resistance to oxidation and degradation at high temperatures. Oils rich in monounsaturated and saturated fats tend to be more stable than those high in polyunsaturated fats.
B. Oil Temperature
Maintaining the optimal oil temperature is perhaps the most critical factor in achieving consistent crispiness. If the oil is too cool, the food will absorb too much oil, resulting in a greasy, soggy product. This happens because the steam barrier, which pushes oil away from the food's surface, is not strong enough to counteract the oil's absorption. Conversely, if the oil is too hot, the exterior will brown and crisp too quickly, potentially burning before the interior is fully cooked, leading to a raw center and a bitter crust. The ideal temperature range for most deep frying is between 325°F and 375°F (160°C and 190°C). Using a reliable thermometer is essential for monitoring and adjusting the oil temperature throughout the frying process.
C. Oil Quality
The quality of the frying oil also significantly impacts the final product. As oil is used, it degrades due to exposure to heat, oxygen, and food particles. This degradation can lead to a lower smoke point, off-flavors, and a reduced ability to create a crispy crust. Old or degraded oil can impart a stale or rancid taste to food and result in a less crispy, more oily product. It's crucial to filter frying oil after each use to remove food particles and store it properly in a cool, dark place. While oil can be reused a few times, it's important to know when to discard it, typically when it darkens significantly, smells off, or foams excessively. Fresh, clean oil is a non-negotiable component for achieving perfectly crispy fried foods.
The outer layer of many fried foods, whether a delicate batter or a robust breading, acts as a crucial 'crispy armor,' playing a pivotal role in achieving that desirable crunch. The composition and application of these coatings are finely tuned scientific endeavors.
A. Batters vs. Breadings
Batters are typically wet mixtures of flour, liquid (water, milk, beer, or carbonated water), and often leavening agents. When food coated in batter hits hot oil, the rapid evaporation of water creates steam, which expands the batter, forming a light, airy, and often bubbly crust. The starch in the flour gelatinizes, creating a rigid structure. The type of liquid used can also influence crispiness; carbonated liquids, for instance, introduce additional air bubbles, leading to a lighter, crispier texture.
Breadings, on the other hand, involve coating food in dry ingredients, often in a multi-step process (e.g., flour, egg wash, breadcrumbs). The flour layer provides a surface for the egg wash to adhere, and the egg wash, in turn, helps the breadcrumbs stick. The breadcrumbs themselves, often made from dried bread, absorb oil and become incredibly crispy as their internal moisture evaporates. The rough surface of breadcrumbs also increases the surface area, promoting more Maillard reaction and thus more flavor and browning.
B. Leavening Agents
In batters, leavening agents like baking powder or baking soda are often employed to create a lighter, more porous, and ultimately crispier coating. When exposed to heat, these agents release carbon dioxide gas, which creates tiny bubbles within the batter. As the batter fries, these bubbles expand, resulting in a less dense, more delicate crust that shatters easily when bitten into. This is particularly effective in tempura-style batters, where a very light and airy coating is desired.
C. Double Frying
For ultimate crispiness and a longer-lasting crunch, the technique of double frying is often employed, particularly for items like french fries. The science behind this method is elegant: the first fry, typically at a lower temperature (around 325°F or 160°C), cooks the food through and begins the process of moisture removal and starch gelatinization. The food is then removed from the oil and allowed to cool. During this cooling period, moisture from the interior of the food migrates to the surface, and the gelatinized starch network sets. The second fry, at a higher temperature (around 375°F or 190°C), rapidly evaporates this surface moisture, creating an intensely crispy exterior. The pre-cooked interior ensures that the food doesn't overcook during this final crisping stage.
D. Gluten-Free Alternatives
For those avoiding gluten, achieving a truly crispy fried coating can be a challenge, as wheat flour's gluten network contributes to structure. However, many excellent gluten-free alternatives can yield superb results. Rice flour, cornstarch, and potato starch are popular choices due to their high starch content and lack of gluten. These flours create a very crisp, almost brittle coating. Blends of these flours, sometimes combined with a small amount of protein (like egg white powder) or a leavening agent, can mimic the texture of traditional coatings while remaining gluten-free. The key is to leverage the gelatinization and dextrinization properties of these alternative starches to build a robust, crispy structure.
Achieving that perfect crispiness in fried foods is only half the battle; maintaining it until the food reaches the plate is equally important. Improper post-frying care can quickly turn a beautifully crispy creation into a soggy disappointment.
A. Draining and Resting
Immediately after removing fried food from the hot oil, it's crucial to drain it properly. Transfer the food to a wire rack placed over a baking sheet lined with paper towels. The wire rack allows air to circulate around all sides of the food, preventing steam from condensing and making the bottom soggy. The paper towels will catch any excess oil that drips off. Avoid placing fried food directly on paper towels or a flat plate, as this traps steam and oil, leading to a greasy, limp result. A brief resting period on the rack also allows any residual oil to drain and the crust to fully set and crisp up.
B. Air Circulation
Air circulation is the enemy of sogginess. As fried food cools, moisture from the interior continues to migrate to the surface. If this moisture has nowhere to go, it will condense on the crispy crust, making it soft and unappetizing. Keeping fried foods on a wire rack ensures that air can flow freely around them, allowing any evaporating moisture to dissipate. If you're frying in batches, it's best to keep the finished pieces warm in a low oven (around 200-250°F or 90-120°C) on a wire rack, rather than stacking them or covering them, which would trap steam.
C. Serving Temperature
While crispiness is a textural attribute, its perception is also influenced by temperature. Fried foods are generally best enjoyed hot, immediately after frying. As they cool, the fats in the oil solidify, and the moisture content within the food can begin to re-distribute, potentially softening the crust. While some foods, like certain types of fried chicken, can retain a good degree of crispiness even when warm, the peak crispy experience is almost always achieved when the food is fresh out of the fryer and properly drained. If reheating is necessary, a hot oven or air fryer is usually preferred over a microwave, as the latter tends to steam the food, leading to sogginess.
VIII. Conclusion: Mastering the Art and Science of Frying
The pursuit of perfectly crispy fried foods is a delicious journey that beautifully marries culinary art with scientific understanding. What might seem like a simple cooking technique is, in fact, a complex interplay of physical and chemical transformations, each contributing to that irresistible crunch and rich flavor.
We've uncovered the critical role of moisture displacement, where hot oil rapidly expels water, creating a dry, rigid crust. We've explored the magic of the Maillard reaction, responsible for the golden-brown hue and the myriad of complex, savory flavors that develop during frying. The structural backbone of crispiness, we learned, is formed by the gelatinization and dextrinization of starches within batters and breadings. Furthermore, the choice and careful management of frying oil, along with the precise chemistry of coatings, are indispensable elements in achieving consistent, desirable results.
Armed with this scientific knowledge, you can move beyond guesswork and apply these principles in your own kitchen. Understanding the importance of patting food dry, maintaining optimal oil temperature, and providing proper post-frying drainage will empower you to consistently produce fried foods that are crispy, flavorful, and free from greasiness. The journey to perfect crispiness is indeed a delicious blend of culinary art and scientific understanding, inviting you to experiment, observe, and savor the satisfying results of your newfound expertise.







